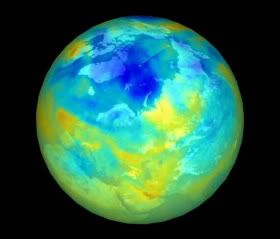
Polar stratospheric clouds (shown) have formed over large areas of the Arctic, which could signal coming losses of protective ozone, new research suggests.
Credit: Ross J. Salawitch/University of Maryland
 Trioxygen, more commonly known as Ozone (O3), is a polar molecule consisting of three oxygen atoms. It is a pale blue gas found throughout the atmosphere, and depending on its location, it can be noxious or beneficial.
Trioxygen, more commonly known as Ozone (O3), is a polar molecule consisting of three oxygen atoms. It is a pale blue gas found throughout the atmosphere, and depending on its location, it can be noxious or beneficial.
In the troposphere (the lowest portion of the Earth’s atmosphere) it is considered a pollutant, and is formed by the reactions of volatile organic compounds (VOCs) and nitrogen oxide gases (NOx) in the presence of sunlight. These compounds and gases are produced by numerous sources, from human-made causes such as industrial emissions and gasoline fumes, to natural phenomenon such as the emission of compounds like isoprene or pinene by different types of trees. Ground-level ozone is harmful to breathe, damaging to crops, and is a primary ingredient of what we call smog.
Beneficial ozone is produced naturally in the stratosphere, particularly the “bottom” portion, commonly called the ozone layer. It serves as a sort of “barrier,” absorbing over 97% of the ultraviolet light emanating from the sun which can be damaging to life on Earth. Ozone depletion (resulting in the formation of ozone “holes”) has a plethora of negative effects on humans, other animals, and vegetation.
Ozone hole around South Pole in 2003. View of the South Pole from NASA’s TOMS (Total Ozone Mapping Spectrometer) satellite. Blue and green indicate relatively large amounts of ozone. Red and yellow mark the “ozone hole”, an area of decreased ozone. (Credit: NASA)
In recent news,
The ozone hole over the south pole is a well-known phenomenon, opening up every spring, letting excess ultraviolet light stream in from the sun and driving up skin cancer risk down under.
But this year, ozone levels at the opposite pole are poised to reach record lows thanks to the right weather patterns and possibly a contribution from the changing climate.
“Don’t panic,” said Markus Rex of the Alfred Wegener Institute for Polar and Marine Research in Potsdam, Germany. “It’s nothing which is a reason for great concern. Still people should have in mind that when they are outside in spring, sunburn times can drop to 20 minutes. People don’t expect that in late March. You wouldn’t expect to get a sunburn in the northern U.S. then.”
While the ozone-depleted area is currently centered over the Arctic, the zone will shift as weather patterns change, and will pass over more populated areas as far south as about 45 degrees north — roughly through Oregon, Minnesota and the New York-Canada border in North America — and possibly even further.
Findings indicate that over the past 2-3 weeks, ozone levels in some areas have already dropped by nearly 50%, and that cold temperatures in the stratosphere and weather patterns will likely drive levels even lower over the next few weeks.
Ozone depletion at both poles is caused by chlorofluorocarbons — commonly known as CFCs and once used in air conditioners and as propellants in aerosol cans — and other ozone-depleting substances, most of which have been phased out thanks to the 1987 Montreal Protocol, but which will persist in the stratosphere for decades to come.
The depletion happens in the stratosphere — the upper atmospheric layer that spans from about 6 to 31 miles above Earth.
CFCs and related chemicals start wiping out ozone once stratospheric temperatures hit about
108 Fahrenheit, low enough to form wispy, shimmering clouds, sometimes called motherof-pearl clouds, in the bone-dry stratosphere. The ice crystals inside these clouds provide surfaces for the reactions that eliminate ozone.These reactions need a third component — sunlight–which is why they get going at this time of year in the Arctic as the sun reappears there.
“This recent winter was a particularly cold winter” in the stratosphere, Rex said, which explains the low ozone levels.
According to Rex and colleagues, however, ozone depletion will be a thing of the past by the end of this century. It is hoped that since CFCs and other ozone-depleting agents are not entering the atmosphere in the quantities they once were, their concentrations will diminish over the years until the ozone holes finally close.



7 comments